The Era of CRISPR/Cas Technology
Mar 18th 2020
- 1. Adaptation:
- 2. CRISPR RNA biogenesis or expression
- 3. Interference
- 1. The wild-type Cas9 (mainly SpCas9 from the bacteria Streptococcus pyogenes and SaCas9 from Staphylococcus aureus), which can cause a site-specific DSB. This results in DSB repair usually by cellular Non-Homologous End Joining (NHEJ) pathway leading to insertions and/or deletions (indels) which disrupt the targeted locus. Alternatively, if a donor template with homology to the targeted locus is supplied, the DSB may be repaired by the homology-directed repair (HDR) pathway allowing for precise replacement mutations to be made.
- 2. The mutant form of Cas9, named Cas9D10A has an inactive RuvC domain, making it a nickase i.e. cleaves only one DNA strand. When “paired nickases” are used with 2 gRNAs, a DSB can be created (Figure 4A). This significantly reduces the off-target effects compared to wild type Cas9.
- 3. Similarly, the H840A mutation inactivates the HNH domain creating a non-target strand-cleaving nickase. H840A is also often used as paired nickases.
- 4. Another variant is a nuclease-deficient Cas9 or dCas9 that incorporates both H840A and D10A mutations. The dCas9 does not possess nuclease activity but can bind to the target DNA. Therefore, it can be used to locate a sequence in the genome without cleavage. By fusing dCas9 with various effector domains, it can also be used as a gene silencing, activation or visualization tool. (Figure 4B).
- 5. Scientists engineered SpCas9 mutants with a substitution of a single positively-charged amino acid residue, from which resulted “enhanced specificity” SpCas9 variants (eSpCas9) which exhibited significantly lower levels of off-target cleavage while having good on-target efficiency. Similarly, high-fidelity SpCas9-HF1 and HypaCas9 has been engineered with improved efficiency.
- 6. Cas12a (Cpf1) is a class 2, type V Cas protein. It requires only a crRNA to guide it to the target but creates staggered cuts with 5-nt 5’ -overhangs as opposed to the blunt cuts generated by Cas9. This mediates a HDR rather than NHEJ. Additionally, Cas12a recognizes T-rich PAM sites and also has lower mismatch tolerance giving rise to lesser off-target effects. Cas12a also has the ability to process its own crRNA, which facilitates the targeting of multiple loci with one pre-crRNA template.
- 7. Cas13a (C2c2) is a class 2, type VI Cas nuclease. It possesses two higher eukaryotes and prokaryotes nucleotide (HEPN)-binding domains, which are ubiquitous in RNases. This gives it the ability to cleave RNA as opposed to the DNA cleaving ability of Cas9 and Cas12a. Cas13a can therefore be harnessed for post-transcriptional repression, with comparable efficiency to RNA interference (RNAi) methods.
The discovery of CRISPR/Cas9 system has revolutionized the landscape of
molecular genetics. CRISPR/Cas9 is a cutting edge gene editing technology that
can make precise cuts in the DNA, in a programmable and directed manner. It has
made gene editing easy, robust and affordable.
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. In 1987, Japanese scientists discovered an unusual DNA structure of alternating repeat and non-repeat sequences in Escherichia Coli. Later, four conserved gene sequences were identified adjacent to these repeats, designated as CRISPR associated genes (Cas). The biological significance of this sequence remained in the dark for almost 20 years. In 2007, a team of scientists made a landmark discovery while studying viral resistance in prokaryotes (Barrangou et al, 2007). CRSPR was identified as a crucial component of the bacterial defence system against bacteriophage infections, a type of adaptive immunity. The discovery initiated a revolution in genetic engineering putting behind the other existing technologies. The CRISPR system was modified to be used a tool to precisely alter genomes in microbes, plants, animals and even humans. The application CRISPR editing spans various domains from basic research to drug discovery and gene therapy. In the recent years, several new and advanced CRISPR systems have been designated to improve efficiency and safety for in-vitro and in-vivo experiments.
CRISPR/Cas immune system in Bacteria and Archae
Since its discovery, the CRISPR was noticed in the genomes of many species of bacteria and archae. The first link between CRISPR and bacterial immunity came in 2005 when two independent research groups of Francisco Mojica in Alicante and Christine Pourcel in Orsay observed that the spacer sequence was homologous to viral DNA. Subsequently, Barrangou et al., in 2007 demonstrated acquired resistance in Streptococcus thermophiles by insertion and deletion experiments of phage DNA in the CRISPR loci. CRISPR is passed to offspring and hence becomes a chronological record of the infections endured by the cell and its ancestors.
The mechanism of CRISPR immunity was delineated when the function of Cas protein was brought to light. Knockdown and rescue experiments emphasized the role of Cas proteins in the process. Different types of CRISPR-Cas systems have been identified based on the variations in Cas genes and different arrangements of CRISPR loci. The initial classification included type I, II and III which had Cas gene specific to Cas3, Cas9 and Cas10 respectively. However, the most recent classification has two classes, Class 1 including type I, III and IV which depend on multiprotein crRNA-complexes for functioning. The Class 2 systems comprise types II, V and VI where a single multi-domain protein is involved (Loureira et al., 2019).
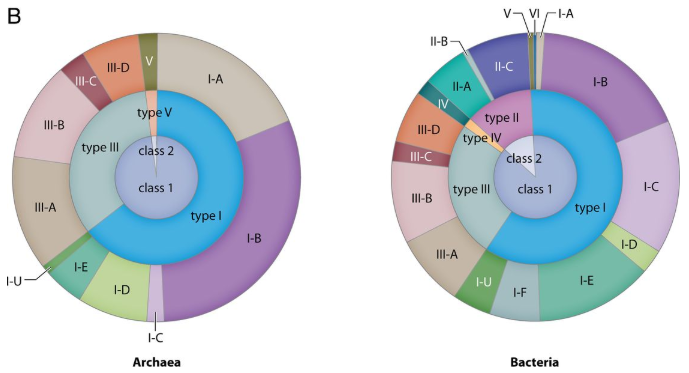
Figure 1: Chart showing the proportions of identified CRISPR-cas loci in the total genomes of bacteria and archaea referred from the literature. (Ishino Y et al., 2018)
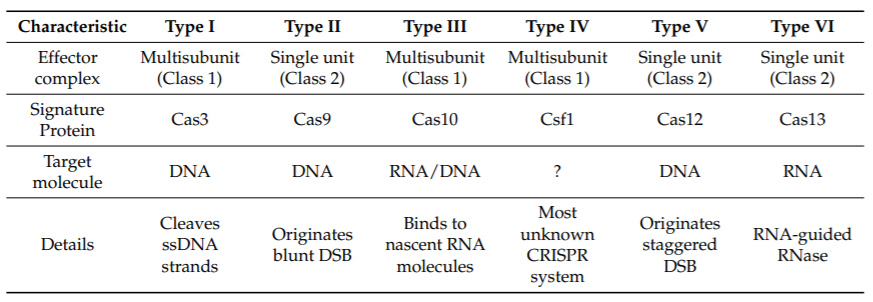
Table 1. Characteristics of different types of clustered regularly interspaced short palindromic repeats (CRISPR) systems. (Loureira et al., 2019)
The mechanism of CRISPR immunity was delineated when the function of Cas protein was brought to light. Knockdown and rescue experiments emphasized the role of Cas proteins in the process. Different types of CRISPR-Cas systems have been identified based on the variations in Cas genes and different arrangements of CRISPR loci. The initial classification included type I, II and III which had Cas gene specific to Cas3, Cas9 and Cas10 respectively. However, the most recent classification has two classes, Class 1 including type I, III and IV which depend on multiprotein crRNA-complexes for functioning. The Class 2 systems comprise types II, V and VI where a single multi-domain protein is involved (Loureira et al., 2019).
The CRISPR-cas mediated defence mechanism is divided into 3 steps (Figure 2).
The genetic material of the invading phage is incorporated into the CRISPR loci. The Cas1 and Cas2 proteins, which are conserved throughout CRISPR-Cas systems, forms a complex that mediates both the excision of protospacer DNA (segment present in the foreign DNA molecule that precedes the spacer sequence) and insertion of the spacer into the CRISPR array. Spacer acquisition requires the recognition of a short motif located near the target sequence, denominated as protospacer adjacent motifs (PAMs). PAMs are short 2–5 nucleotide sequences, specific to each CRISPR-Cas subtype and bacteria, which determine the spacer alignment within the CRISPR array.
The CRISPR locus is transcribed into pre-crRNA which is processed by the type-specific Cas endonucleases into mature crRNAs. In type II systems, a small trans-activating RNA molecules (tracrRNA) is transcribed which is complimentary to the repeat sequence. It contains 3 stem-loop hairpin structures and binds to the pre-crRNA (Figure 3). Cas9 stabilizes the tracrRNA:pre-crRNA interaction and together forms a complex for later recognition and cleavage of pre-crRNA by RNase III for complete processing.
When infected, the crRNAs-Cas endonuclease effector complex is recruited to cleave the foreign DNA or RNA in a sequence-dependent manner. In type II systems, the Cas9 is guided by the dual RNA complex tracrRNA:crRNA. Binding of this dual RNA causes the activation of Cas9. The complex screens for the correct PAM site in foreign genetic elements, which will be opposite to the target strand. When identified, the dsDNA is unwound and crRNA binds to the target resulting in a double stranded break (DSB) 3 nucleotide upstream of the PAM site.
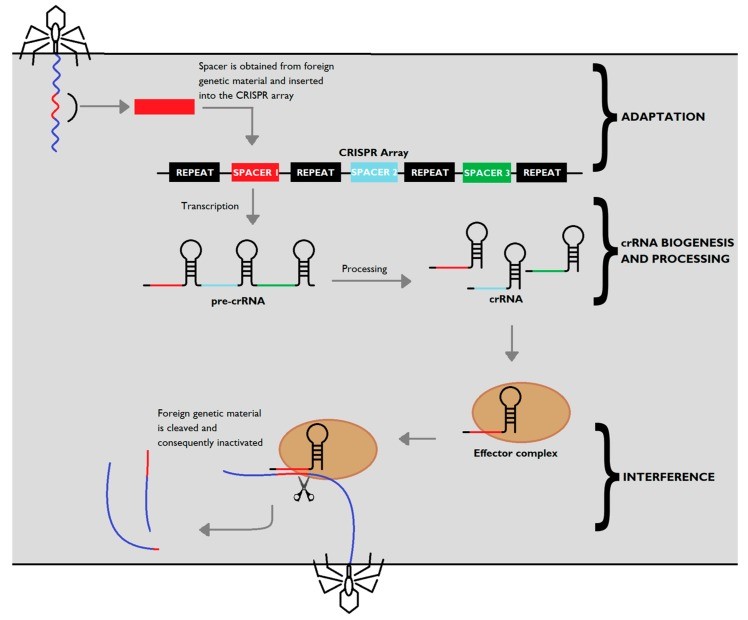
Figure 2: CRISPR-Cas adaptive immunity. Upon injection of genetic material from a virus or a plasmid into the bacteria, part of the invading sequence is cleaved and incorporated into the CRISPR locus, forming a new spacer within the locus. The CRISPR array is transcribed into a precursor to crRNA molecules (pre-crRNA), which is then cleaved into mature crRNA, which form effector complexes with type-specific Cas proteins (brown). When a foreign sequence matches a CRISPR spacer, the matching crRNA binds to the invading strand, activating Cas proteins with nuclease activity which silence the invader. . (Loureira et al., 2019)
The possibility of exploiting CRISPR for gene editing in Cas-containing systems was first hinted by Makarova et al. in 2006, in their study trying to understand the CRISPR mechanism. Finally, in June 2012, Jinek, Doudna and colleagues made a landmark publication demonstrating the use of Type II systems for successfully silencing the target GFP gene in E.coli. The following years witnessed a boom in CRISPR/Cas9 applications. Various research groups showed that CRISPR/Cas9 can be used for gene insertions, deletions, substitutions and also gene regulation by activating or repressing transcription. CRIPSR/cas9 gained widespread approval and soon emerged as a popular tool for gene manipulation in bacteria to cell lines to multicellular organisms.
CRISPR/Cas9 for genetic engineering
Class 2 CRISPR/Cas systems became the choice for developing genome editing technology due to their simpler structure and mechanism involving small RNA molecules and a single Cas protein.

Figure 3: Cleavage mechanism of target DNA by crRNA-tracrRNA-Cas9. The Cas9-crRNA-tracrRNA complex binds to foreign DNA containing PAM, where Cas9 binds and starts to unwind the double strand of the foreign DNA to induce duplex formation of crRNA and foreign DNA. Cas9 consists of two regions, called the REC (recognition) lobe and the NUC (nuclease) lobe. The REC lobe is responsible for nucleic acid recognition. The NUC lobe contains the HNH and RuvC nuclease domains and a C-terminal region containing a PAM-interacting (PI) domain. The HNH domain and the RuvC domain cleave the DNA strand, forming a duplex with crRNA and the other DNA strand, respectively, so that double-strand break occurs in the target DNA. (Ishino Y et al., 2018)
Different modified versions of Cas9 nucleases and other Cas proteins nuclease have been developed to improve efficiency and adapt to various applications in gene editing.
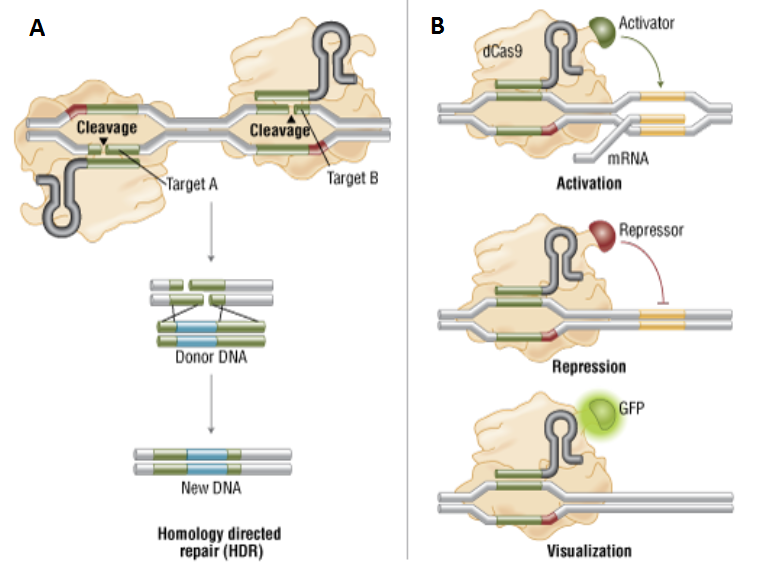
Figure 4: Variants of Cas9
A) Genome engineering by double nicking
with paired Cas9 nickases.
B) Localization with dCas9 and dCas9 fusion proteins.
Advantages and Applications of CRISPR technology
Genome editing using CRISPR/Cas9 system has now become a staple in most labs. The technology has been employed in all the major scientific areas from microbiology to animal models to agriculture and medicine (Figure 5). When compared to the earlier technologies, CRISPR/Cas offers a simpler and affordable solution (Table 2). One of the major advantages is that Cas9 systems depend solely on the engineering of short gRNA molecules, without the need for laborious and costly protein programming and validation and therefore saving time and resources. As mentioned earlier, Cas9 systems can also be easily multiplexed by delivering multiple gRNAs to the cells.
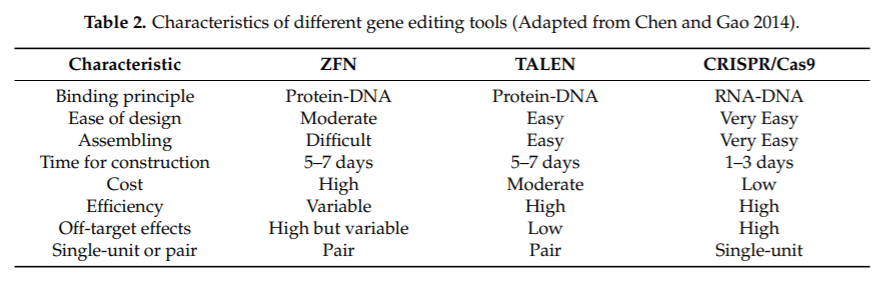
Table 2: Characteristics of the commonly used gene editing tools. (Chen and Gao, 2014)
Another pro is the better compatibility with most of the common delivery vectors when compared to ZFNs and TALENs which require vectors with higher load capacity. All these reasons contributed to the popularity and widespread adoption of the CRISPR/Cas9 as a mainstream gene manipulation tool.
Apart from using CRISPR as a tool to generate and study disease models (cell lines and animals), there is a growing interest in using CRISPR as a therapeutic tool. Experiments are being conducted for in-vivo CRISPR-mediated knockout of defective genes. For example, knockout of NANOG and NANOGP8, genes lead to significant suppression of prostate cancer in mice. Moreover, clinical trials for CRISPR-mediated editing of human T-cells is being conducted for treatment of multiple myeloma, sarcoma and melanoma. CRISPR is also being tested to correct genetic disorders like cystic fibrosis, blindness and sickle cell anemia apart from eliminating several viral and bacterial infections.
What Next?
The discovery of CRISPR/Cas has truly been a feat in molecular biology. In the span of 30 years we have witnessed how the adaptation of a simple bacterial mechanism have revolutionized the gene editing technology. It is the simplicity and versatility of the system that has led to its immediate acceptance in the scientific community.
The applicability of CRISPR-Cas systems has moved beyond just DNA cleavage. This user friendly technology has become a gene manipulation, gene regulation, visualization, diagnostic and therapeutic tool. Certain ethical concerns are also being raised in creating engineered food, treating genetic disorders in humans and creating mutant species. Researchers are putting in constant efforts to reengineer and improve efficiency and safety while overcoming some of the limitations like off-target effects. However, the full potential of CRISPR/Cas is yet to be unfolded. The results of the upcoming clinical trials will be a testimony to the immense power of this transformative technology.
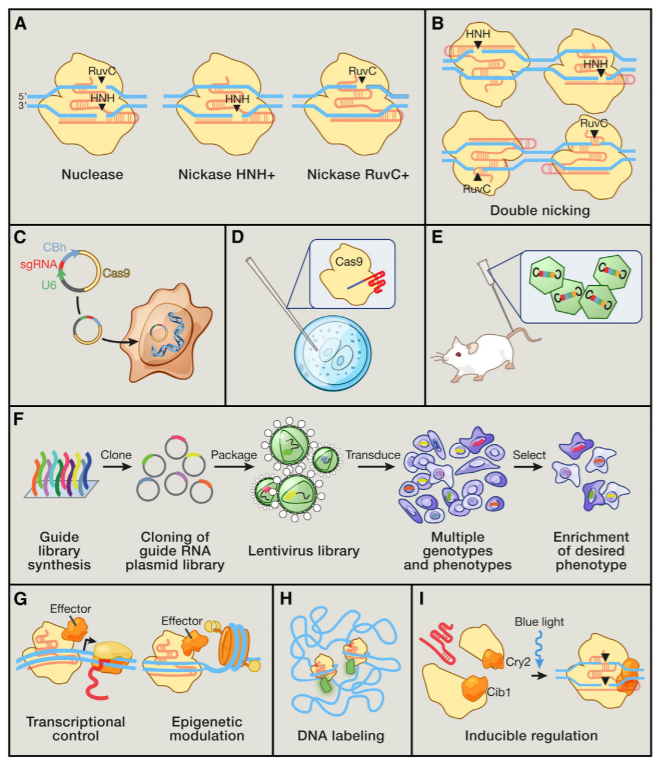
Figure 5: Applications of Cas9 as a Genome Engineering Platform (A) The Cas9 nuclease cleaves DNA via its RuvC and HNH nuclease domains, each of which nicks a DNA strand to generate blunt-end DSBs. Either catalytic domain can be inactivated to generate nickase mutants that cause single-strand DNA breaks. (B) Two Cas9 nickase complexes with appropriately spaced target sites can mimic targeted DSBs via cooperative nicks, doubling the length of target recognition without sacrificing cleavage efficiency. (C) Expression plasmids encoding the Cas9 gene and a short sgRNA cassette driven by the U6 RNA polymerase III promoter can be directly transfected into cell lines of interest. (D) Purified Cas9 protein and in vitro transcribed sgRNA can be microinjected into fertilized zygotes for rapid generation of transgenic animal models. (E) For somatic genetic modification, high-titer viral vectors encoding CRISPR reagents can be transduced into tissues or cells of interest. (F) Genome-scale functional screening can be facilitated by mass synthesis and delivery of guide RNA libraries. (G) Catalytically dead Cas9 (dCas9) can be converted into a general DNA-binding domain and fused to functional effectors such as transcriptional activators or epigenetic enzymes. The modularity of targeting and flexible choice of functional domains enable rapid expansion of the Cas9 toolbox. (H) Cas9 coupled to fluorescent reporters facilitates live imaging of DNA loci for illuminating the dynamics of genome architecture. (I) Reconstituting split fragments of Cas9 via chemical or optical induction of heterodimer domains, such as the cib1/cry2 system from Arabidopsis, confers temporal control of dynamic cellular processes. (Hsu et al., 2014)
Chen L. , Tang L., Xiang H., Jin L., Li Q., Dong Y., Wang W. and Zhang G. Advances in genome editing technology and its promising application in evolutionary and ecological studies. GigaScience 2014, 3:24.
Gasiunas G., Barrangou R., Horvath P. and Siksnys V. (2012). Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Pnas 109, E2579–E2586.
Hale C.R., Zhao P., Olson S., Duff M.O., Graveley B.R., Wells L., Terns R.M. and Terns M.P. (2009). RNA-Guided RNA Cleavage by a CRISPR RNA-Cas Protein Complex. Cell 139, 945–956.
Hsu P.D., Lander E.S. and Zhang F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 157, June 5, 2014 ª2014 Elsevier Inc.
Ishino Y, Krupovic M, Forterre P. 2018. History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J Bacteriol 200:e00580-17.
Jiang F. and Doudna J. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017. 46:505–29.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J.A., and Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821.
Loureiro A. and da Silva G. CRISPR-Cas: Converting A Bacterial Defence Mechanism into A State-of-the-Art Genetic Manipulation Tool. Antibiotics 2019, 8, 18; doi:10.3390/antibiotics8010018.
Makarova K.S., Grishin N.V., Shabalina S.A., Wolf Y.I., Koonin E.V. (2006). A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biology Direct 2006, 1:7.
Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10(10):957–963. doi:10.1038/nmeth.2649
Rath D., Amlinger L., Rath A, Lundgren M. The CRISPR-Cas immune system: Biology, mechanisms and applications. Biochimie Vol 117, October 2015, Pages 119-128
Scott T., Urak R., Soemardy C. & Morris K.V. Improved Cas9 activity by specifc modifcations of the tracrRNA. Scientific Reports (2019) 9:16104
Zhan T., Rindtorff N., Betge J., Ebert M.P., Boutros M. CRISPR/Cas9 for cancer research and therapy. Seminars in Cancer Biology Vol 55, April 2019, Pages 106-119.
https://www.broadinstitute.org
Written By Shalitha Sasi
(Molecular biologist and pharmaceutical R&D scientist)
