Trends in Gene Therapy
Jan 29th 2020
DNA modification is not new to the scientific world. Advances in recombinant DNA technology has enabled successful gene modifications in microbes, plants and animals, implying the great potential to alter human genes. However, until recently, gene editing in humans as a cure for genetic disorders has mostly remained in the lab. Gene therapy is as bio-therapeutic strategy which involves transfer of DNA into a patient’s target cells to alter gene expression in order to correct a defective gene or a gene product that causes a disease. Since the first experiment in 1989, gene therapy has been an active area of research. After three decades of rigorous experimentation and facing numerous roadblocks, gene therapy is finally finding its way into the clinic. Currently, at least twenty gene therapy products have been approved and more than two thousand are in clinical trials.
Most of the approved gene therapy drugs target single-gene mutations for rare diseases with limited treatment. Another factor underlying the successful clinical translation of certain gene therapies is the ease of administration in target tissues, for example, diseases of the eye and hematopoietic system. A better understanding of diseases at a molecular level and the evolution in gene editing technology has opened doors the various possibilities for safer and more effective human gene editing strategies.
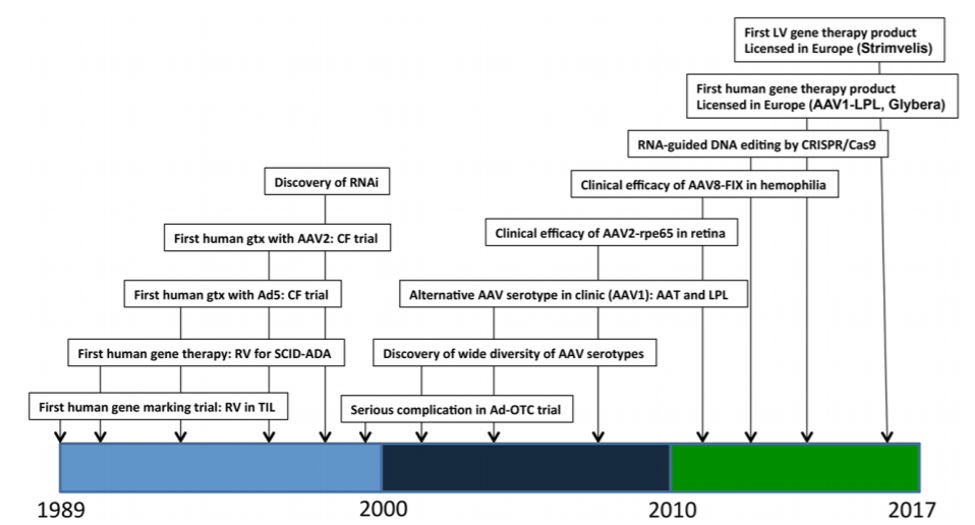
Figure 1: Timeline of major events in clinical gene therapy. A few selected key dates in the history of human gene therapy are depicted, with the dates indicated on the x-axis. AAV2, adeno-associated virus type 2; Ad5, adenovirus type 5; CF, cystic fibrosis; CRISPR, clustered regularly interspaced short palindromic repeats; FIX, clotting factor IX; LPL, lipoprotein lipase; OTC, ornithine transcambamylase; RNAi, RNA inhibition; RPE65, 65 kilo-Dalton retinal pigment epithelial protein; RV, gammaretrovirus; SCID-ADA, severe combined immune deficiency due to adenosine deaminase deficiency; TIL, tumor-infiltrating lymphocytes. Reference: AM Keeler et al., 2017
How is it done?
The main approaches to gene therapy are gene replacement, gene inactivation, gene addition or gene editing to either compensate for a defective gene or to make a functional protein. The gene transfer process usually requires a carrier, known as a vector, to deliver DNA or RNA into specific cells in the body. Most gene therapies use modified viruses as vectors, as they can introduce the genetic material by infecting the cell. More recently, the CRISPR/Cas9 gene editing technology has made it possible to modify chromosomal DNA and repair genetic errors directly. Based on the mode of administration of the gene therapy agent, the process can be categorized into two types (figure 2).
- 1.In-vivo gene therapy
- This involves direct injection of the vector into the body. Depending upon the vector and the target, in vivo gene therapy can be administered intravenously, injected into the muscles, infused or injected into an organ, or injected directly into a tumor site.
- 2.Ex-vivo gene therapy
- Here, the cells are removed from the body, genetically modified in the lab and then re-infused back into the patient. The cells can be from the same patient (autologous) or from a donor (allogeneic). These modified cells can replicate and spread in the body for a long term beneficial effect. Furthermore, the ex vivo strategy allows the genetic alteration of the targeted cell subpopulation without affecting other cells or organs.
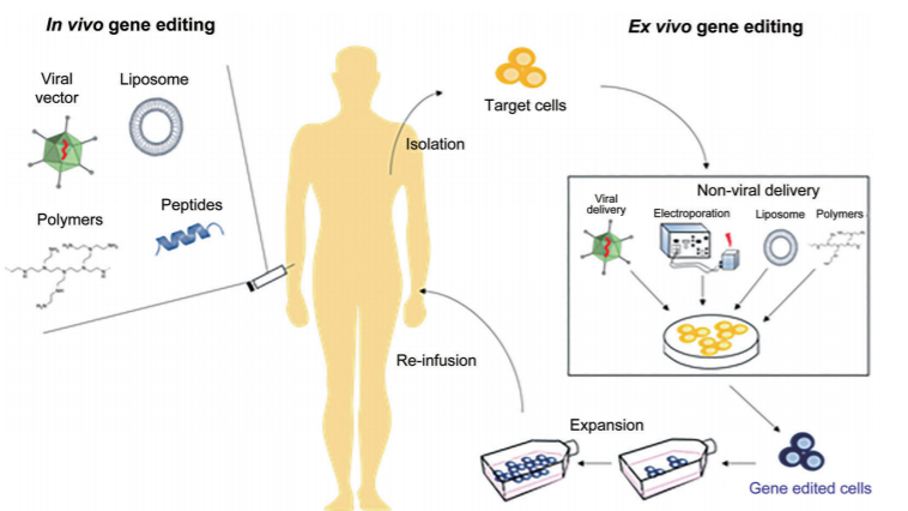
Figure 2: Schematic of approaches to gene therapy applicable to both gene editing and gene transfer. Reference: Shim et al., 2017 Gene therapy: an overview of approved and pipeline technologies. Ottawa: CADTH; 2018 Mar. (CADTH issues in emerging health technologies; issue 171).
Different technologies
A safe, effective and controllable gene delivery system is the key step in gene therapy. The different types of vectors include viral and non-viral options.
Viral vectors
The viruses used for gene therapy are rendered non-pathogenic by replacing their disease causing gene with the DNA of interest whilst retaining their infectious properties. The choice of vector depends on the size of the gene to be inserted, the target cells (dividing or non-dividing, and cell type), host genomic integration and immunogenicity. The following are some of the most widely used viral vectors.
- Retroviral vector (RV) is one of the earliest vector to be used in gene therapy. The genetic material is transcribed into DNA and randomly integrated into the host genome, ensuring stable and prolonged gene expression. The downside of this option is the risk of generating dangerous replicable viruses and insertion mutations in the host due to random integration.
- Herpes simplex viruses (HSV) are large enveloped dsDNA viruses characteristic of their lytic and latent nature of infection, which result in life-long latent infection of neurons and allows for long-term transgene expression. Deletion of HSV genes has generated expression vectors with low toxicity and an excellent packaging capacity of >30 kb foreign DNA.
- Lentiviral vector (LV) is a modification of the HIV-1 virus. It is one of the most common tool used due to its longer length of the gene fragment, high transfection efficiency, high compatibility, wide infection scope, and low immunogenicity. The lentiviral short-hairpin RNA (shRNA) vectors are now preferred over the small interfering RNAs (siRNA).
- Adenovirus vector (AV) is a non-enveloped virus with double-stranded linear DNA. It has a high transfection efficacy and a large packing capacity. However, the drawback is it only expresses the target gene transiently and can induce an immunogenic response.
- Adeno-associated virus (AAV) is a non-pathogenic a protein shell surrounding a small, single-stranded DNA of ~ 4.8 kb, initially discovered as a contaminant of adenovirus preparations. AAV is dependent on co-infection with other viruses, mainly adenoviruses, for replication. These viruses can insert genetic material at a specific site on chromosome 19 with near 100% certainty. Owing to their potential to mediate stable, long-term gene expression and as they don’t produce an immune response, AAVs have shown to be safe and effective in many preclinical and clinical studies. Some of the limitations include low capacity and difficulties in producing it.
Non-viral vectors
Non-viral vectors have multiple advantages over viral vectors like higher genomic load capacity, low immunogenicity, good biodegradability and the option of large scale production. Moreover, they also can avoid being degraded by nucleic acid enzymes with high electric potential and specific surface area. However, due to the lack of cell-targeting capability and low gene transfection rate in vivo, most of them are currently used for in vitro operations. Some of the non-viral vectors are:
- Naked DNA or Plasmids is the simplest method of non-viral transfection. Clinical trials carried out of intramuscular injection of a naked DNA plasmid have shown very low success rate because of low cellular internalization. More efficient methods for delivery have been developed such as electroporation and gene gun (which shoots DNA coated gold particles into the cell using high pressure gas).
- Oligonucleotides (ONs) are used to inactivate the genes involved in the disease process. Most ON therapies use antisense mechanisms to disrupt the transcription of the faulty gene. Few examples are gapmers, steric block ONs, antagomirs, small interfering RNAs (siRNAs), micro-RNA mimics, and splice switching ONs. ONs have also been designed to bind to Toll-like receptors to forming aptamers that have a completely different mode of action (Lundin et al.; 2015).
- Liposomes/Lipoplexes are DNA molecules covered by a lipid membranes to enhance transfection and protect DNA from nucleases. Cationic lipids are the most commonly used as they readily complex with the negative charged DNA and can easily interact with the cell membrane. Liposomes can be digested by lysosomes or endocytosed to release the DNA into the cytoplasm.
- Polyplexes are complexes of polymers with DNA, most of which consist of cationic polymers. Unlike liposomes polyplexes cannot release their DNA load into the cytoplasm, sand therefore requires co-transfection with endosome-lytic agents such as inactivated adenovirus. Of late, polymers such as polyethylenimine, chitosan and trimethylchitosan have been developed that incorporates endosome disruption.
- Nanoparticles provide a new approach to gene vectors by wrapping or adsorbing DNA or RNA on the surface. Specific targeted molecules such as monoclonal antibody are coupled on the particle surface which allows nanoparticles to be endocytosed or phagocytosed by cells. . Some special nanomaterials have magnetism, optics, and thermal performance, which can achieve delivery and controlled release of targeted genes.
Gene editing
In this method, rather than transferring modified genes, vectors are used to transfer the gene editing machinery directly into target cells. These “programmable nucleases” can make precise and direct changes in the cell genome to bring about the desired outcome. Unlike viral vectors, which may have a transient effect and supplement missing or defective genes, gene editing technologies can be used to add, inactivate, or correct a gene with a permanent effect. Gene editing nucleases bind to DNA with varying degrees of specificity and produce double stranded breaks (DSBs). These breaks are then repaired by the cell’s own DNA repair mechanisms. The repair mechanisms include homologous recombination, which requires a homologous template which is endogenously or exogenously supplied (Figure 3). The nicks may also be repaired without a template, resulting in non-homologous end joining (NHEJ) or microhomology-mediated end joining (MMEJ). These cellular DNA repair mechanisms are utilized for different gene editing goals. NHEJ is more useful for excising part of the genome or inactivating a gene, while homologous recombination with an exogenously supplied template can be utilized to create gene insertions or make base pair substitutions, and is more applicable for repairing genetic mutations. The major types of nucleases used for gene editing are meganucleases, ZFNs, TALENs and the CRISPR/cas9 system.
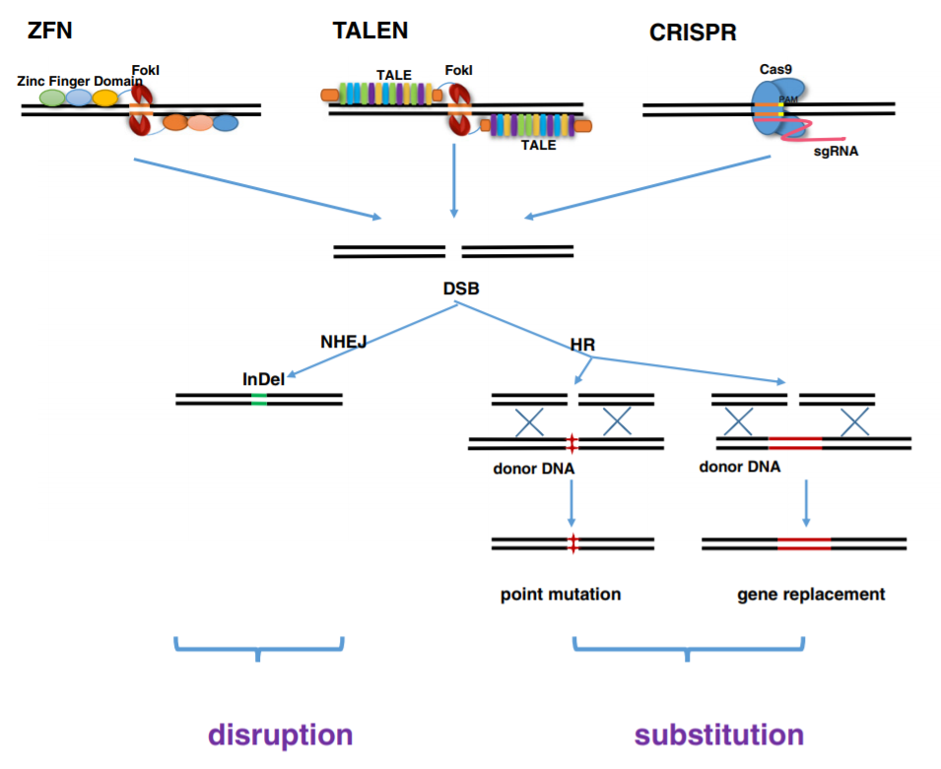
Figure 3: ZFN, TALEN and CRISPR/Cas9 achieve precise and efficient genome modification by inducing targeted DNA DSBs, which would be corrected by NHEJ and HR repair mechanisms. NHEJ-mediated repair leads to the introduction of variable length insertion or deletion. HR-mediated repair could lead to point mutation and gene replacement, in the present of donor DNA. Reference: Chen et al., 2014
- Meganucleases (MGNs)
- Meganucleases, or homing endonucleases are endodeoxyribonucleases that can recognize a large DNA sequence (>12 base pairs) in living cells. They can induce site-specific DSBs, and thereby stimulate homologous recombination of up to 10 000-fold in cultured cells in-vitro. MGN-induced DSB can also be repaired by non-homologous end-joining (NHEJ). By modifying their recognition sequence through protein engineering, the targeted sequence can be changed. One of the major downside of the technology is that MGNs could potentially cleave off-target sequences, resulting in toxicity.
- A recent advancement is a combinatorial approach in DNA binding domain from transcription activator-like (TAL) effectors is incorporated to create a hybrid nuclease (Figure 4). These "megaTALs" combine the ease of engineering and high DNA binding specificity of a TAL effector with the high cleavage efficiency of meganucleases.
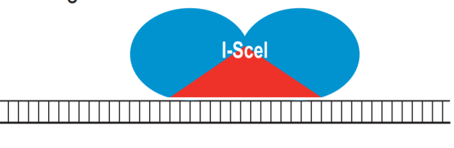
Figure 4: The hybrid meganuclease, I-SceI, is shown bound to its DNA target. The catalytic domain, which also determines DNA sequence specificity, is shown in red. Reference: Voytas et al., 2014
- Zinc finger nucleases (ZFN)
- ZFNs were the first programmable nucleases that was designed to cleave at predetermined genomic loci to create double stranded breaks (DSBs). These consist of a DNA binding domain and a DNA cleavage domain. The DNA binding domain is a zinc finger protein, a transcription factor that can recognize a trinucleotide DNA sequence. This ZFP is fused to an endonuclease called FokI via a linker. FokI endonuclease works as a dimer, therefore DSBs occur only at sites of binding of two ZFNs to the opposite DNA strands (Figure 5). The target site specificity can be improved by increasing the number of ZF motifs fused to the FokI. The higher the specificity of the ZFPs, the lower the ZFNs off-target cleavage, and hence toxicity. Another method to lessen ZFN toxicity is to use ZF nickases that cleave at only one predetermined DNA strand of a targeted site. ZFN nickases are produced by inactivating the catalytic domain of one monomer within the ZFN pair (Chandrasegaran et al., 2017). ZFNs are generally delivered into host cells using AAV or LV constructs. Apart from toxicity and low in-vivo efficiency, another major drawback of ZFN is the cost of production.
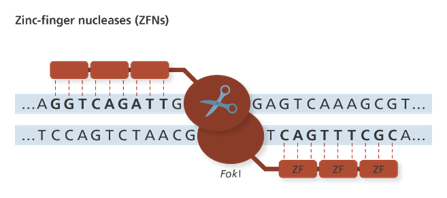
Figure 5: Mechanism of action of ZFNs
- Transcription activator-like effector nucleases (TALENs)
- TALEN is another engineered nuclease, which demonstrates better specificity and efficiency than ZFN. Similar to ZNFs, TALENs have DNA binding motifs linked to FokI endonuclease (Figure 3). But unlike ZFNs, each TALE domain can recognize a single nucleotide enhancing the specificity. However, the major challenge for TALEN is to clone the large modules in series, joint these modules in designed order by ligase in an efficient way.
- CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats)
- The latest approach employs the bacterial immune defense system based on clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated genes (Cas). The CRISPR/Cas9 used in gene editing comprises of the Cas9 nuclease, trans-activating crRNA (tracrRNA) and a single guide RNA (sgRNA). Recognition of the DNA site in the CRISPR-Cas9 system is controlled by complimentary base pairing on sgRNA. However, the specificity of the system depends on the protospacer adjacent motif (PAM) located immediately downstream of the target sequence. The CRISPR/Cas9 has many advantages over ZFNs and TALENs, including easy design for any genomic targets, easy prediction regarding off-target sites, and the possibility of modifying several genomic sites simultaneously (multiplexing).
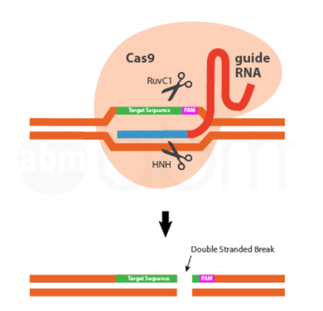
Figure 6: The last 20 base pairs of the gRNA acts as a homing device, recruiting the Cas9 endonuclease to the appropriate target sequence. Once there, the two cleavage domains of the Cas9 create a double stranded break 3 or 4 base pairs downstream of the PAM sequence. The DSB is then repaired by either the error-prone non-homologous end joining repair pathway, or the template-dependent homology directed repair.
- Moreover, RNA is easier and cheaper to synthesize than the protein domains used with the ZFNs and TALENs. However, one big barrier is delivering all the components required to complete the editing process. Certain mications of Cas9 have been reported to limit the off-target effects. One is the use of transient expression delivery systems, whereas another suggestion is to alter the Cas9 half-life. A number of Cas9 variants with lower off-target specificity have been developed, including HF-Cas9, eCas9, and HypaCas9 (Maji B et al., 2017) (Chen et al., 2017). Furthermore, CRISPR-Cas12 (Cpf1) and CRISPR-Cas13a (C2c2), have been developed which are new variants of Cas9 that can recognize different PAMs, providing a higher on-target specificity. Another interesting alternative is a Cas9 fusion with FokI nuclease, which combines the advantages of ZFNs/TALENs and the CRISPR-Cas9 system.
- Therapeutic approaches with CRISPR/Cas9 have already been demonstrated positive results for genetic diseases like for Duchenne muscular dystrophy and liver disease fumarylacetoacetate hydrolase deficiency and numerous therapies are currently in trials.
The road ahead
Gene editing technologies including CRISPR, ZFN and TALEN allows precise genomic modifications in the human genome, which has paved way for modern medicine. In the last few years a number of gene therapies based on gene editing, especially CRISPR system, have entered human clinical trials. The gene therapy market is categorized into cancers, neurological diseases, rare genetic diseases, cardiovascular disorders, and infectious diseases. Currently, cancers and monogenic diseases dominate the gene therapy research and development. the year 2018 witnessed gene therapy approval for monogenic diseases, inherited blindness, certain inherited neurodegenerative diseases, metabolic genetic disorders and a number of bone marrow and lymph nodes cancers (Figure 7).
When it comes to clinic, the viral vectors (mainly retrovirus, lentivirus, adenoassociated virus) has exhibited a better performance in terms of efficiency, specificity and reduced administration dose. However, other safety and efficacy concerns prevail such as prolonged laboratory procedures for conducting clinical studies, unknown product interactions with host, and high cost which are some hurdles that need to be overcome.
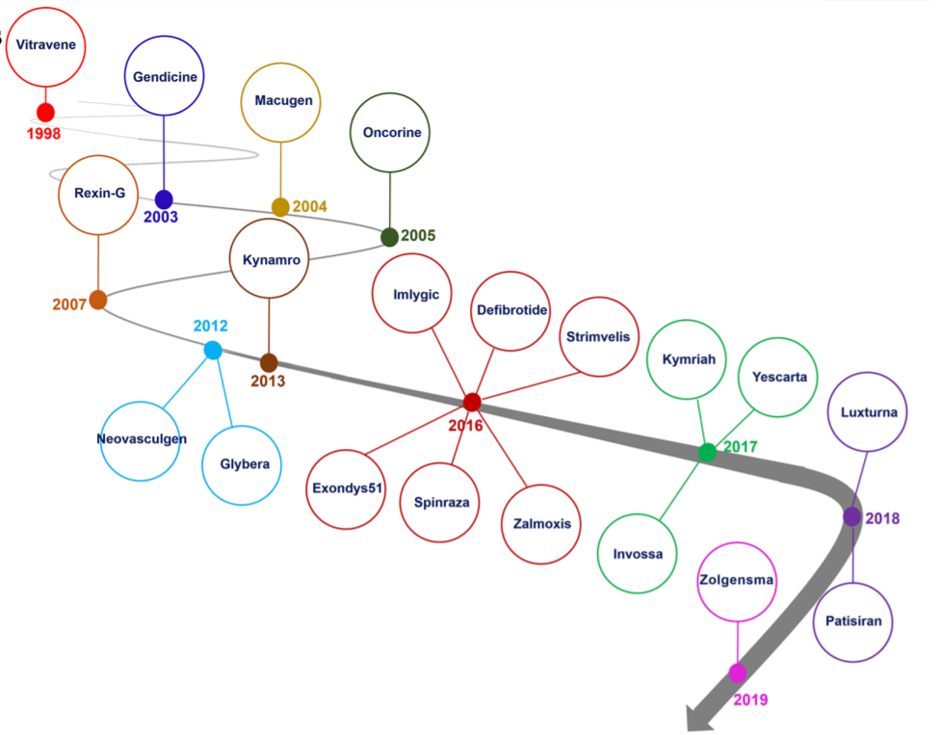
Figure 7: Gene therapy market began with approval of Vitravene drug in 1998, and it was continued with approval of Zolgensma drug in 24 May, 2019. 2016 and 2017 years were promising points in gene therapy market since near 10 gene therapy products such as Imlygic, Defibrotide, Spinraza, Zalmoxis, Exondys51, Strimvelis, Invossa, Yeskarta and Kymriah were approved by relevant authority. Reference: Shahryari et al, 2019
With the influx of multiple gene therapy candidates, there is also a rising need to address the legal and ethical policies involved. Genetic Modification Clinical Research Information System (GeMCRIS),developed by the NIH and FDA, is a database designed to assist in tracking gene therapy products, monitor trends in the field, and provide transparency through a public-facing website.
This new wave of gene therapy has revolutionised the treatment of genetic disorders. Even though, gene therapy is still in the developmental phases, there has been great progress in the last decade promising a future that can eradicate genetic defects humans.
References
Alengo Nyamay’Antu, Maxime Dumont, Valérie Kedinger & Patrick Erbacher. Non-Viral Vector Mediated Gene Delivery: the Outsider to Watch Out For in Gene Therapy. Cell & Gene Therapy Insights. Doi: 10.18609/ Cgti.2019.007
Alison Sinclair, Saadul Islam, Sarah Jones. Gene therapy: an overview of approved and pipeline technologies. Ottawa: CADTH; 2018 Mar. (CADTH issues in emerging health technologies; issue 171).
AM Keeler, MK ElMallah and TR Flotte. Gene Therapy 2017: Progress and Future Directions. : Clin Transl Sci (2017) 10, 242–248; doi:10.1111/cts.12466
Chapdelaine, P., Pichavant, C., Rousseau, J. et al. Meganucleases can restore the reading frame of a mutated dystrophin. Gene Ther 17, 846–858 (2010) doi:10.1038/gt.2010.26
Chen, J. S. et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550, 407–410 (2017).
Francis S. Collins, M.D., Ph.D., and Scott Gottlieb, M.D. The Next Phase of Human Gene-Therapy Oversight. N Engl J Med 379;15 nejm.org October 11, 2018
Giulliana Augusta Rangel Gonçalves1 , Raquel de Melo Alves Paiva. Gene therapy: advances, challenges and perspectives. Einstein. 2017;15(3):369-75 DOI: 10.1590/S1679-45082017RB4024
Lei Chen, Linyi Tang, Hui Xiang, Lijun Jin, Qiye Li, Yang Dong, Wen Wang and Guojie Zhang. Advances in genome editing technology and its promising application in evolutionary and ecological studies. GigaScience 2014, 3:24
Lundin KE, Gissberg O, Smith CI. Oligonucleotide Therapies: The Past and the Present. Hum Gene Ther. 2015;26(8):475–485. doi:10.1089/hum.2015.070.
Lundstrom K. Viral Vectors in Gene Therapy. Diseases. 2018;6(2):42. Published 2018 May 21. doi:10.3390/diseases6020042
Maji, B. et al. Multidimensional chemical control of CRISPR-Cas9. Nat. Chem. Biol. 13, 9–11 (2017).
Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi:10.1126/science.1232033
Shahryari A, Saghaeian Jazi M, Mohammadi S, Razavi Nikoo H, Nazari Z, Hosseini ES, Burtscher I, Mowla SJ and Lickert H (2019) Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 10:868. doi: 10.3389/fgene.2019.00868
Srinivasan Chandrasegaran. Recent advances in the use of ZFN-mediated gene editing for human gene therapy. Cell Gene Ther Insights. 2017 ; 3(1): 33–41. doi:10.18609/cgti.2017.005.
Sun W, Shi Q, Zhang H, Yang K, Ke Y, Wang Y, Qiao L. Advances in the techniques and methodologies of cancer gene therapy. Discov Med. 2019 Jan;27(146):45-55.
Voytas DF, Gao C (2014) Precision Genome Engineering and Agriculture: Opportunities and Regulatory Challenges. PLoS Biol 12(6): e1001877. doi:10.1371/journal.pbio.1001877
https://ghr.nlm.nih.gov/primer/genomicresearch/gen...
www.channelnewsasia.com/news/world/2019-the-year-g...
www.genengnews.com/insights/crispr-jump-starts-gen...
Written By: Shalitha Sasi
