Solid phase sandwich ELISA for the determination of Human Tenascin-C containing high molecular weight variants (Human Tenascin-C HMWV) in serum, plasma (heparin), or cell culture media. For research use only, not for use in diagnostic procedures.<br><br>
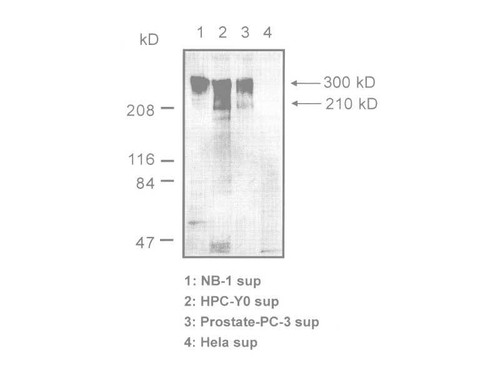
Tenascin-C is an extracellular matrix (ECM) glycoprotein that is composed of 210-400 kDa subunits consisting of four domains. One subunit has a TA domain at the N-terminal end, then an epidermal growth factor-like sequence domain (EGF-like domain), a fibronectin type III(FN III) repeat domain, and a fibrinogen-like domain at the C-terminal end. There is an alternatively spliced domain in the FN III domain, and it generates some types of variants of Tenascin-C. The subunits form a trimer by twisting at the N-terminal coiled domain and form a hexamer by a disulfide bond, in tissue. While low molecular weight variants of Tenascin-C are present in normal tissue, it is said that high molecular variants of Tenascen-C is expressed in various diseased tissue including cancer.
- Assay Description:
- 1 hour incubation (37°C) + 30 min. (4°C) + 30 min. (RT) = 2 hours total incubation time
- Catalog number:
- 27751
- configuration:
- 96 Determinations, 12x8 removable strips
- controls:
- None provided
- design:
- Solid phase sandwich ELISA using 2 kinds of high specific monoclonal antibodies
- FDA Status:
- For research use only, not for use in diagnostic procedures
- MSDS:
- notes:
- The protocol for this product (see above) is intended to serve as an example only. Please refer to the Instructions For Use provided with the assay kit for precise details.
- Protocol:
- Sample types:
- Serum, plasma (heparin), or cell culture media
- Sample volume:
- 100 μL of properly diluted unknown / determination
- standards:
- 8 standards, serially diluted from 1 prepared lyophilized standard
- Standard range:
- 0 / 0.38 - 24 ng/mL
- storage:
- 2 - 8 °C
- sensitivity:
- 0.01 ng/mL
- Species:
- Human
- Additional info:
- References: